Ostomy Bags Manufacturing Plant Project Report 2026
Table of Contents
With the aging of the global population, the rising incidence of colorectal cancer, and the continuous growth in the number of patients with inflammatory bowel disease (IBD), the ostomy bags manufacturing market is entering a stage of steady expansion. More and more companies and enterprises are beginning to choose to enter the ostomy bags manufacturing market, and at the same time, they will also need a professional, specific and executable ostomy bags manufacturing plant project report .
Zodelo, as a professional stoma care brand, are based on 30 years of industry accumulation. Systematically sort out the construction requirements, technical routes, investment budgets and market outlooks of global ostomy bags manufacturing factories. This report is intended for medical device investors, industrial plant planners, medical consumables brand owners, OEM/ODM factories, etc. Provide authoritative, structured and executable ostomy bags manufacturing plant project report 2026.
About Ostomy Bags

The first item of the ostomy bags manufacturing plant project report is to specifically analyze and understand what is ostomy bags.Ostomy bag is a medical device specially designed for patients undergoing stoma surgery, used to collect the excrement discharged from the stoma. When there is inflammation, cancer or trauma in the patient’s intestines or anus. If the patient is unable to defecate normally, the doctor will connect a part of the patient’s own colon to the abdominal wall and make an incision in the abdomen. Feces can be discharged into the colostomy bag through the stoma. The ostomy bag can collect the excreted feces and urine, effectively protect the skin around the stoma, and help patients maintain personal hygiene and cleanliness for a long time.
1. The composition of Ostomy Bags
- Bag body: Ostomy bag body is usually made of transparent or opaque soft materials and is used to collect the excrement discharged from the opening. It features strong leak-proof performance, strong odor resistance, and skin-friendly softness.
- Skin barrier: Basically made of hydrocolloid or silicone material, it is adhered around the stoma skin to fix the bag body, which can prevent excrement from contaminating the skin and prevent leakage.
- Discharge outlet: The discharge outlet is usually located at the bottom of the Ostomy bag. The open discharge outlet can discharge loose or liquid waste, which can be emptied, cleaned and reused.
- Ostomybag accessory: Activated carbon filter, which can absorb odors and prevent them from leaking. Leak-proof rings and leak-proof paste are used to achieve stronger sealing and adhesion, preventing leakage and odor.
2. Properties of ostomy bags
Understanding properties of ostomy bags is also an important item in the ostomy bags manufacturing plant project report.
- Good sealing property:The skin barrier of ostomy bag will form a closed system with the skin. It can effectively protect the skin, prevent odor and leakage, and reduce the risk of skin inflammation.
- Skin-friendliness:skin barrier is made of hydrocolloid and is skin-friendly, effectively protecting the skin. The bag body is soft, skin-friendly and has low allergenicity.
- Good odor control:Most ostomy bags use activated carbon filtration devices to help exhaust and adsorb odors. ostomy bags
- Concealment: The ostomy bag body is usually made of matte, soft and low-noise multi-layer film. It is invisible and lightweight for daily wear.
- Emptying convenience:It features an easy-to-open, easy-to-clean, and resealable bottom opening. It is used for quickly emptying the excrement inside the bag.
- Adaptation flexibility:Patients can freely combine one-piece ostomy bag, two-piece ostomy bag, flat skin barrier and convex skin barrier according to their own needs. In terms of flexible combination.

3. Ostomy Bags Manufacturing Process
Understanding properties of ostomy bags is also an important item in the ostomy bags manufacturing plant project report.
- Good sealing property:The skin barrier of ostomy bag will form a closed system with the skin. It can effectively protect the skin, prevent odor and leakage, and reduce the risk of skin inflammation.
- Skin-friendliness:skin barrier is made of hydrocolloid and is skin-friendly, effectively protecting the skin. The bag body is soft, skin-friendly and has low allergenicity.
- Good odor control:Most ostomy bags use activated carbon filtration devices to help exhaust and adsorb odors. ostomy bags
- Concealment: The ostomy bag body is usually made of matte, soft and low-noise multi-layer film. It is invisible and lightweight for daily wear.
- Emptying convenience:It features an easy-to-open, easy-to-clean, and resealable bottom opening. It is used for quickly emptying the excrement inside the bag.
- Adaptation flexibility:Patients can freely combine one-piece ostomy bag, two-piece ostomy bag, flat skin barrier and convex skin barrier according to their own needs. In terms of flexible combination.
Ostomy Bags Manufacturing Process
The production process of ostomy bag must comply with ISO 13485 and GMP requirements. The specific production process is as follows:
Multilayer Film Extrusion
The film of Ostomy Bags is generally co-extruded from multiple layers such as EVA, PE, and PA. It has odor-blocking performance, waterproof and leak-proof properties as well as mechanical toughness.
Hydrocolloid Barrier Formulation
It is basically composed of CMC, pectin, butyl rubber, etc. Through machine mixing, hot pressing forming, cooling, die-cutting is carried out to create a stomp hole or to be self-cut by the user.
Bag Fabrication
The main production steps are:
- Precision die-cutting
- HF welding / heat sealing
- Reserve the structure of exhaust holes and sewage outlets
- Install an activated carbon filter
Convex Baseplate Molding
Convex Baseplate is formed by injection molding and then bonded and pressed with a hydrocolloid.
Ostomy Bag System Assembly
One piece ostomy bag: The bag body and the base are directly heat-sealed
Two piece ostomy bag: The flange system is made, and a high-precision snap-on structure is required
Materials of ostomy Bags

- Ostomy bag film:Various composite films such as PE, EVA,PA,EVOH,PVDC, etc.
- Skin barrier: CMC, pectin, gelatin, butyl rubber and other materials.
- Connecting ring (double-piece type) : PP,PE.
- Flange Activated Carbon filter:Activated Carbon,PET, non-woven fabric coating layer.
- Closure System:Velcro,Clamp, heat-sealed folding structure.
Applications of Colostomy Bags
- Colorectal cancer:Colorectal cancer can cause intestinal stenosis or even complete obstruction, requiring the removal of the rectal lesion to establish a permanent stoma.
- Inflammatory bowel disease (IBD) :such as Crohn’s disease, ulcerative colitis, etc., these diseases can cause repeated inflammation, ulcers and even gastrointestinal perforation.
- Intestinal obstruction:If acute intestinal obstruction caused by inflammation or tumor temporarily makes the patient’s intestine unusable, a temporary colostomy is required, and a temporary colostomy bag should be used to prevent intestinal perforation.
- Intestinal damage caused by trauma: such as car accidents, knife wounds, gunshot wounds, etc., prevent the injured area from being further contaminated and deteriorated. Colostomy is needed to bypass the damaged area to allow it to rest and heal.
- Congenital malformations: such as congenital megacolon, congenital anal atresia, and the inability of newborns to excrete feces normally. colostomy surgery intervention is needed to help restore excretory function
Key Features of the Ostomy Bags Manufacturing Plant Project Report

A comprehensive, professional and executable Ostomy Bags Manufacturing Plant Project Report needs to include the following points:
1. Market Overview
Global & Regional Demand Forecast (2026-2030)
Competitive landscape (mainstream brands, OEM, ODM)
Market pricing system and profit range
Technology Trends and Regulatory Requirements (FDA/CE/MDR/ISO 13485)
2. Product Portfolio
- Single-piece/double-piece manufacturing pockets
- Drainable/Closed-end ostomy bag
- Flat/convex chassis
- Types of bag body membrane structure
- Hydrogel formula
- Filtering component
- Accessory system
3. Ostomy Bag Manufacturing Process
- Co-extrusion of multi-layer composite films
- Hydrogel mixing and tabletting
- High-frequency heat sealing of the bag body
- Flange injection molding
- Component automated assembly
- EO/Gamma sterilization
- Quality Inspection (QA/QC)
- Sterile packaging
4. List of Key Equipment
- Equipment model
- Unit price
- Functional requirements
- Energy consumption and maintenance costs
- Degree of automation
5. Capital & Operating Costs
- Investment in fixed assets
- Equipment depreciation
- Raw material costs (films, colloids, flanges, filters, etc.)
- Labor cost
- Sterilization and quality inspection costs
- Inventory management overhead
- And conduct a Break-even Analysis.
6. Profitability Analysis
- Gross profit margin, net profit margin
- Product line comparison revenue
- OEM/ODM/self-owned brand revenue model
- Analysis of Market Price Fluctuation sensitivity
7. Risk Analysis
- Regulatory and certification risks
- Risk of rising raw material prices
- The risk of intensified competition
- Automation equipment failure/supply chain risks
- Risk of changes in market demand
Historical, Current, and Forecasted Price Trends of the Ostomy Bags Manufacturing market Historical Price Trends
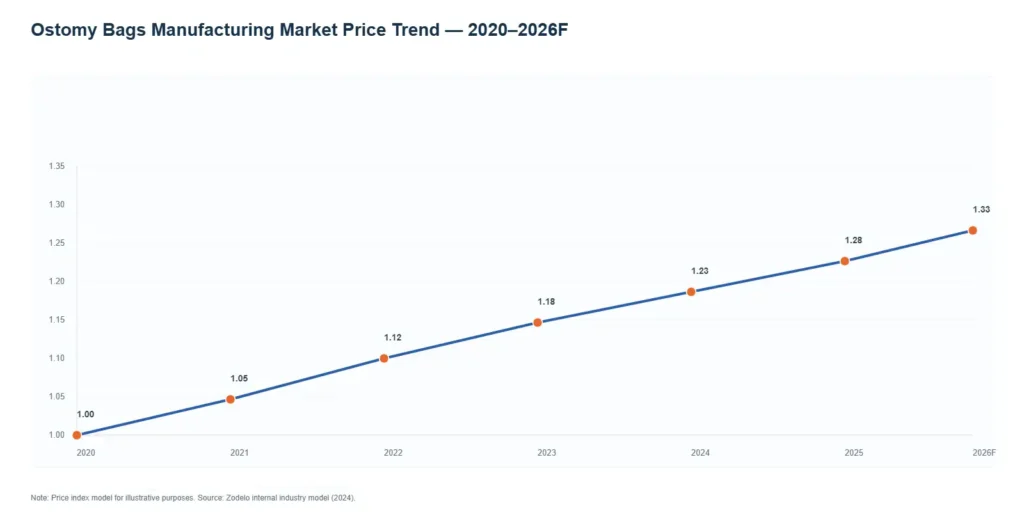
Historical Price Trends:
- 2010-2015:Ostomy Bag products were mostly low/mid-range, and the unit prices were generally low. The raw materials are relatively stable, and the compression of profits mainly comes from competition.
- 2016-2020:The popularization of multi-layer barrier films and improved hydrocolloid processes, the upgrading of Ostomy Bag products led to an increase in the proportion of mid-to-high-end products, and the average unit price rose slightly; At the same time, the intensification of OEM/ODM competition has led to a decline in prices in some regions.
- From 2020 to 2023: Due to the impact of the pandemic and global logistics, transportation costs and material fluctuations (films, activated carbon) have pushed up manufacturing costs. Most manufacturers offset costs by enhancing automation and optimizing formulas, but retail prices have risen in the short term.
Current Price Trends:
- The markets in North America and Europe can accept higher prices for individual items (the unit price of high-end products with smart or special skin protection can reach $1.5-4.0). The price range in Latin America and Southeast Asia remains low ($0.25-0.80).
- Ostomy bagproducts with active filtering and low allergenicity can generate a premium of 20-60%.
- OEM price pressure persists: Large medical groups and distributors are sensitive to prices, and bulk purchases have driven down unit prices. However, they have high requirements for quality and compliance, and factories need to invest more in certification costs.
Forecast Price Trends
- Conservative scenario (low growth) : Global supply chain costs return to normal, but market competition intensifies, with an average annual price fluctuation of approximately -1% to +1%.
- Neutral scenario (baseline) :Technological upgrades (automation + material optimization) offset the increase in raw materials and energy consumption, with the average annual price remaining stable or rising slightly by 1-3%.
- High-growth scenario (popularization of high-end and intelligent products) : The penetration rate of high-end ostomy bag products increases and smart manufacturing bags are launched. The overall weighted average price increases by 3-6% per year, but the prices of low-end products may be depressed.
Conclusion: Analyzed through the Ostomy Bags Manufacturing Plant Project Report. For Ostomy Bag manufacturer, long-term profits come more from the product differentiation and brand premium of ostomy bag rather than relying solely on cost leadership.
Financial Investment Overview of Ostomy Bags Manufacturing Plant Project Report

1. Revenue and cost calculation
Annual total Revenue:
Unit price × 18,000,000 pieces = $9,000,000
(Calculation: 0.50 × 18,000,000 = 9,000,000)
Total Variable Cost:
Unit variable cost: $0.30 × 18,000,000 = $5,400,000
(Calculation: 0.30 × 18,000,000 = 5,400,000)
Annual Contribution profit = revenue – variable cost
= $9,000,000 – $5,400,000 = $3,600,000
Annual Operating Profit = contribution profit – fixed operating expenses
= $3,600,000 – $1,000,000 = $2,600,000
Operating margin as % of revenue
Gross profit margin = Operating profit/revenue = $2,600,000 / $9,000,000
First, calculate the numerator divided by the denominator: 2,600,000/9,000,000 = 0.288888… ≈ 28.89%
Simple Payback period (excluding time value)
Payback period = Initial CAPEX/annual operating profit = $4,500,000 / $2,600,000
Calculation: 4,500,000 ÷ 2,600,000 = 1.730769230769… Approximately 1.73 years
Note: The above payback period is a very idealized “static payback period”. For real projects, factors such as depreciation, taxes, interest, working capital (receivables/payables/inventory), initial marketing expenses, and sales collection cycles need to be taken into account.
2. Main financial KPIs
- Unit Manufacturing Cost (Unit COGS)
- Production line Capacity Utilization
- Yield/First Pass Yield (%)
- Inventory Turns
- OEE (Overall Equipment Effectiveness)
- Gross profit margin/Net profit margin/payback period/IRR (If a discounted cash flow model is adopted)
Profit Margins and Pricing Strategies of Ostomy Bags Manufacturing Plant Project Report

1. The basic profit range within the Ostomy Bags Manufacturing industry
OEM/ Bulk purchasing: Gross profit margin is often between 10% and 30% (competition and thin profit)
Mid-range brand products: Gross profit margin 25% – 40%
High-end brand: Gross profit margin 40-60% or even higher (depending on patents/formulas/channels)
2. Pricing Strategies of Ostomy Bags Manufacturing Plant Project Report
- Cost-plus pricing
Final selling price = Total unit cost × (1 + target gross profit margin)
Applicable: Mature products, customers sensitive to prices, OEM business.
Advantages: Simple and easy to calculate; Disadvantage: Ignoring the market’s willing price.
- Value pricing
Pricing is based on the value that patients/medical institutions are willing to pay (more comfort, fewer complications, and extended bag change cycles).
Applicable to: High-end or hypoallergenic products.
Advantages: It can achieve higher gross profit margins; Disadvantage: It requires investment in market education and evidence (clinical trials, user research).
- Tiered Pricing/Channel Pricing
Pricing is differentiated by region and channel (high price in developed markets and low price in developing countries; direct sales are higher than distribution).
Applicable to: Global brand strategy.
- Subscription/Care-as-a-Service
Provide consumables + online care service packages (e.g., monthly delivery + care consultation subscription).
Applicable to: DTC (Direct-to-Consumer) model, enhancing customer stickiness and smoothing revenue.
- Penetration Pricing
Enter new markets at low prices to seize market share, and later raise prices or lead to paid services.
Risks: Possible brand damage and high upfront subsidy costs.
Regulatory Frameworks and Environmental Considerations of Ostomy Bags Manufacturing Plant Project Report

1. Regulatory Frameworks of Ostomy Bags Manufacturing Plant Project Report
Judicial district | Regulations/Requirements (Core) | Product Classification (Example of Making a pocket | Key compliance points (Direct impact on the manufacturing plant |
European Union (EU | MDR 2017/745 (EU Medical Device Regulation) | Mostly Class IIa (possibly IIb depending on the purpose/risk) | Technical File, clinical evaluation (CER), UDI, EUDAMED registration, manufacturer obligations (PMS, Vigilance), compliance with ISO 13485 recommendations |
The United States (FDA | 21 CFR Part 820 (QSR); Product path: 510(k) or PMA/De Novo (high risk) | Mostly Class II (generally through 510(k)) | QMS (compliant with QSR), 510(k) comparison type performance data, UDI, registration/listing, and biocompatibility data in combination with ISO 10993 |
Canada | SOR/98-282 Medical Device Regulations; MDALL | Similar to the EU/US classification system | Licensing and quality system (CMDCAS/ISO 13485) and clinical/performance evidence |
Britain | UK MDR 2002 + UKCA conversion requirements | Similar to the EU (independent management after the transition period) | UKCA marking, registration, and PMS requirements |
China (Pre-export compliance) | National Medical Products Administration (NMPA) (Registration-Related | Medical disposable devices | Biocompatibility, production quality management, and registration technical data (if necessary) |
2. Environmental Considerations of Ostomy Bags Manufacturing Plant Project Report, for details, please refer to the following content.
Environmental factors | Risk/Control points required | Suggested engineering/management controls | Compliance/reporting requirements |
Organic solvents/VOCs | Vocs are emitted during the preparation or cleaning process of hydrocolloids | Use low-volatile solvents or solvent recovery systems; Sealed mixing room | Local emission permit Air quality monitoring |
EO waste gas | The exhaust gas from EO sterilization contains residual EO and volatile substances | EO waste gas condensation/combustion/activated carbon adsorption treatment | Exhaust gas emission permit + Regular monitoring |
Hazardous chemicals | Additives, fuels, fungicides, etc | SDS management, hazardous waste classification, qualified carriers | Record of hazardous waste declaration and disposal |
Wastewater | Wastewater containing solid or chemical pollutants is produced from cleaning and process washing | Wastewater pretreatment + biochemical treatment or outsourcing sewage treatment | Local emission standards and monitoring reports |
Solid waste | Waste films, waste packaging materials, and expired consumables | Classified recycling, cooperation with recycling plants, and reduced design | Recyclable waste statistics, ESG reports |
Energy consumption (Cleanroom | HVAC and cleanroom systems consume a lot of energy | Heat recovery, variable frequency fans, intelligent control, renewable energy | Energy consumption monitoring and carbon emission accounting (Scope 1-3) |
Packaging waste | Disposable outer packaging is difficult to recycle | Research on lightweight design, recyclable materials, and alternative materials | Comply with the EU packaging Directive/local regulations |
Analysis of the of Ostomy Bags Manufacturing Plant Project Report by Key Country
According to Ostomy Bags Manufacturing Plant Project Report,the key countries are analyzed as follows.
| Country/Region | Market size and growth | Payment and price range | Registration/compliance difficulty | Manufacturing costs and supply chains | Bidding and channel accessibility | Gross profit potential | Main risks | Suggested entry strategy |
| The United States | high | high | high | Medium-low | Medium to high | high | High responsibility risk and fierce competition | High-end two-piece model +DTC/ subscription; Nearshore manufacturing Strengthen RWE |
| The European Union (Germany/France/Northern Europe) | Medium to high | Medium to high | Very high | Medium-low | Medium | Medium to high | MDR compliance is emphasized, and bidding prices are lowered | Sustainability + Differentiation in dermatology; High automation and ESG |
| China | high | In the | In the | high | high | In the | Centralized procurement and price wars | Dual product lines (basic + high-end); Localized supply chain |
| India | Medium to high | Low – medium | In the | high | Medium | In the | Quality system and brand building cycle | First, increase mid-range traffic +KOL education; Gradually high-end |
| Brazil/Latin America | In the | In the | Medium to high | Medium | Medium | In the | Exchange Rate and Tariff | Local joint venture/assembly; Strong distribution cooperation |
| GCC(Gulf and the Middle East) | In the | Medium to high | In the | Medium | Medium | Medium to high | Policy and geopolitical fluctuations | Component import + local assembly; Education and services on the government side |
FAQs - Ostomy Bags Manufacturing Plant Project Report
1.How much capital is needed to start a colostomy bag production factory?
Total investment: 8-15 million US dollars equivalent (including factory renovation, equipment, verification, registration and first-year operation)
2.How can I start a colostomy bag manufacturing enterprise?
Total investment: 8-15 million US dollars equivalent (including factory renovation, equipment, verification, registration and first-year operation)
3.What raw materials are needed to produce manufacturing bags?
- Hydrocolloid back adhesive (CMC, pectin, gelatin and additives)
- Multilayer barrier films (PE/PA/EVOH, etc.)
- Non-woven fabric/skin-friendly backing material
- Activated carbon/filter membrane module (deodorization and venting)
- Flange/snap ring (PP/ABS/TPE)
- Adhesives/release paper/protective film, packaging materials
- Sterilization-related consumption (EO trays/indicator cards, etc., if self-built)
4.What machinery and equipment are needed to start a pocket-making factory?
- Water colloid batching and coating drying line
- Film co-extrusion or lamination machine (or external film collection + dry lamination)
- Precision die-cutting machines, punching machines
- Ultrasonic/heat sealing equipment, automatic assembly machine
- Youdaoplaceholder6 Online leak/seal strength tester
- Cleanroom HVAC and environmental monitoring
- Packaging line (forming – filling – sealing), coding and serialization
- EO or gamma sterilization capability (self-built or outsourced)
5.What licenses and approvals are needed to start an ostomy bag manufacturing plant?
- Quality system: ISO 13485; Risk Management ISO 14971; Packaging ISO 11607; Biocompatibility ISO 10993; Sterilization ISO 11135/11137
- Regulatory registration: FDA 510(k)/ EU MDR CE/ China NMPA registration certificate, etc
- Local production license, cleanroom/environmental impact assessment and fire compliance, UDI and traceability filing
6. Which are the world's top ostomy bag manufacturer?
- Zodelo (China):Professional and flexible customization, strong technical research and development capabilities, factory wholesale prices
- Coloplast (Denmark) : Renowned for the SenSura Mio series, emphasizing skin fit and comfort; Clinical standardization and high trustworthiness.
- Hollister (USA) :A century-old brand, leading in patient education
- ConvaTec (UK) :With years of clinical experience, its products are widely used in hospital channels.
- Braun (Germany) : Precision manufacturing and high-end medical systems
7. What is the cost structure of establishing an ostomy bag manufacturing plant?
Typical proportion: 40-55% of materials; Labor: 8-15%; Manufacturing and energy consumption: 10-15%; Quality control and compliance: 5-8%; Packaging and logistics 8-12%; The remaining portion of depreciation and administrative expenses.
8. How long does it take for an ostomy bag manufacturing plant to break even?
The common duration is 18 to 36 months
Depends on the registration path, yield rate/capacity ramp-up, channel distribution speed and product structure (the higher the proportion of high-end products, the faster the return on investment).
More Resourses about Ostomy Bags Manufacturing Plant Project Report
- Colostomy— NHS
- Top 10 Colostomy Bag Manufacturers And Suppliers In The World 2026– zodelo
- Top 10 Colostomy Bag Manufacturers And Suppliers In China 2026– zodelo
- Colostomy Bags Manufacturing Plant Project Report 2025: Industry Trends, Plant Setup, Machinery, Raw Materials, Investment Opportunities, Cost and Revenue – imarcgroup
- Colostomy Bags Manufacturing Plant Project Report 2025 – expertmarketresearch

Top 10 Urostomy Bag Brands in 2026
Top 10 Urostomy Bag

Convex Wafer vs Flat: What’s the Difference
Convex Wafer vs Flat

Top Ileostomy Bag Brands in 2026
Top Ileostomy Bag Br
Ostomy Paste vs Ring: What’s the Difference
Ostomy Paste vs Ring