Brand and Company Profile
ZODELO is a professional brand created by Suzhou Kang Youwei Medical Technology Co., Ltd. for the ostomy care field. Since 2016, we have focused deeply on real patient needs and continuously innovated in product design, baseplate formulas and venting structures.
With our own manufacturing facility and a strict quality management system, ZODELO products cover multiple ranges from economical to premium. In the ostomy care segment of China’s e-commerce platforms, ZODELO has achieved leading sales performance and strong user reputation.
The company has passed ISO 13485 quality management system certification and obtained CE certification for related products, providing a solid foundation for global market entry and long-term cooperation.
Brand Vision and Positioning
ZODELO’s brand philosophy is “Care for a Better Life”. We are committed to providing ostomy patients with more comfortable, safer and more reassuring solutions. We not only focus on the products themselves, but also on patients’ long-term quality of life and emotional well-being.
Globally, we seek responsible, experienced and long-term oriented partners to build a highly competitive international ostomy care brand together.

Product Portfolio and Core Strengths

Ostomy Pouch Series: one-piece and two-piece ostomy pouches in various sizes and opening styles.

Ostomy Baseplates: different materials and formulas to fit various skin types and stoma shapes.

Ostomy Accessories: ostomy powder, skin barrier film, skin barrier rings, ostomy paste, elastic barrier strips, etc.
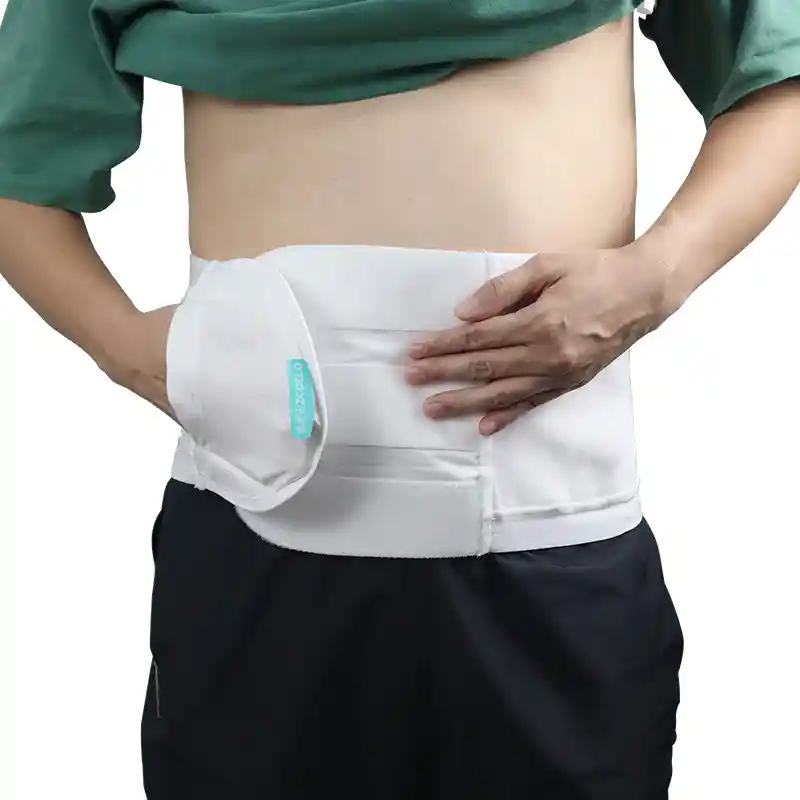
Support and Fixation: ostomy support belts, fixation belts, elastic abdominal belts and similar products.
Our key strengths include:
- Comprehensive product lines: covering different clinical needs and payment capabilities from entry-level to high-end.
- In-house manufacturing: strong flexible production capacity, supporting OEM/ODM and brand export.
- Robust quality system: strict implementation of medical device standards with full batch traceability.
- Proven domestic market: extensive real user feedback from the Chinese consumer market to support continuous improvement.
- Ongoing R&D investment: continuous optimization of venting design, leakage prevention and skin-friendliness.
Cooperation Model and Distribution Levels
To serve global ostomy bag markets more efficiently, ZODELO adopts a multi-tier distribution model: “Country/Regional Master Distributor + Regional Distributor + Terminal Partners”.The specific scope of authorization and detailed terms shall be subject to the final signed agreement between ZODELO and each partner.
Level 1 – Country/Regional Master Distributor
- Responsible for overall market development, team building and channel management in a given country or group of countries;
- May enjoy conditional exclusivity in the authorized territory once agreed conditions are met;
- Responsible for developing and managing Level 2 distributors and taking primary responsibility for brand image and positioning in the territory.
Country/Regional Master Distributor – Qualification Criteria
- Holds valid licenses and permits for medical device distribution in the relevant territory.
- Has at least 3 years of experience in medical consumables, ostomy care, wound care or related fields.
- Possesses a sales network that covers major hospitals, clinics, pharmacies or specialty channels.
- Has a stable sales team and at least one dedicated product manager / clinical support staff.
- Accepts ZODELO’s brand and compliance requirements and cooperates with due diligence reviews.
- Is able to fulfill the agreed first order and annual purchase targets, which are typically equal to 3–4 months of expected sales volume.
Level 2 – Regional Distributor
- Responsible for provinces, states or key cities within a country;
- Executes regional sales and promotion plans under the master distributor’s coordination;
- May receive relative exclusivity or priority protection in the assigned region.
Regional Distributor – Qualification Criteria
- Has a base of hospital/clinic/pharmacy customers in its region.
- Has the capability for routine inventory management and regular customer visits.
- Accepts the unified pricing system and brand standards set by the master distributor and ZODELO.
- Can achieve the agreed regional annual sales target and basic initial order amount.
Level 3 – Terminal Partners (Dealer / Pharmacy / Clinic / Online Partner)
- Includes dealers, pharmacies, clinics, rehabilitation centers and online channel partners;
- Focus on terminal sales and patient service, implementing unified pricing and brand guidelines;
- Normally not granted regional exclusivity; performance is mainly evaluated by service quality and execution at the point of sale.
Terminal Partners – Qualification Criteria
- Able to provide basic product information and after-sales support to patients.
- Complies with ZODELO’s display and pricing rules and refrains from malicious price cutting.
- Maintains good cooperation and communication with upstream distributors and provides feedback from the market.
Pricing Policy and Ordering Requirements
The following is a typical commercial framework. Specific figures and discounts will be determined on a case-by-case basis during business negotiations, depending on the market characteristics of each country.
1. First Order and Annual Targets
- First order: determined according to the target market size and first-year sales goal, typically equivalent to 3–4 months of expected sales;
• Annual purchase target: clearly defined for each product line in an appendix to the agreement, with quarterly breakdown;
• Target achievement will be linked to the maintenance of exclusivity, rebates and marketing support.
2. Payment Terms and Credit Policy
- The first order is usually paid by 100% T/T in advance;
• After the cooperation reaches a certain scale and stability, an appropriate credit limit or payment term may be discussed based on risk assessment;
• All payment terms are subject to the final signed contract.
3. Inventory and Turnover Requirements
- Master distributors are advised to maintain a safety stock level of at least 2 months of average sales;
• Regular sharing of inventory and sales data is encouraged so that the factory can plan production and shipments more efficiently;
• Key SKUs and high-turnover items should be prioritized to avoid stock-outs.
4. Rebates and Marketing Support
- Depending on annual performance, a sales rebate or marketing support fund of around 2%–4% of the purchased amount may be granted;
• Marketing funds are mainly used for academic conferences, patient education activities, online promotion, etc., and require appropriate supporting documents for reimbursement.
Brand Building and Marketing Support
The following is a typical commercial framework. Specific figures and discounts will be determined on a case-by-case basis during business negotiations, depending on the market characteristics of each country.
1. First Order and Annual Targets
- First order: determined according to the target market size and first-year sales goal, typically equivalent to 3–4 months of expected sales;
• Annual purchase target: clearly defined for each product line in an appendix to the agreement, with quarterly breakdown;
• Target achievement will be linked to the maintenance of exclusivity, rebates and marketing support.
2. Payment Terms and Credit Policy
- The first order is usually paid by 100% T/T in advance;
• After the cooperation reaches a certain scale and stability, an appropriate credit limit or payment term may be discussed based on risk assessment;
• All payment terms are subject to the final signed contract.
3. Inventory and Turnover Requirements
- Master distributors are advised to maintain a safety stock level of at least 2 months of average sales;
• Regular sharing of inventory and sales data is encouraged so that the factory can plan production and shipments more efficiently;
• Key SKUs and high-turnover items should be prioritized to avoid stock-outs.
4. Rebates and Marketing Support
- Depending on annual performance, a sales rebate or marketing support fund of around 2%–4% of the purchased amount may be granted;
• Marketing funds are mainly used for academic conferences, patient education activities, online promotion, etc., and require appropriate supporting documents for reimbursement.
Training, Quality and After-Sales Service
1. Training Support
- Regular online/offline training on product knowledge, clinical application and sales techniques;
- Professional introductions and demonstrations for key customers, nurses and clinical staff;
- Assistance in organizing regional training and educational events when needed.
2. Quality and Complaint Handling
- ZODELO is committed to strict quality control under the relevant quality management systems to ensure consistent product quality;
- For end-user complaints, distributors should collect information in a timely manner and report back to ZODELO so that issues can be analyzed and resolved as quickly as possible;
- In cases involving serious quality or safety concerns, product recalls and corrective actions will be carried out in accordance with ZODELO procedures.
Cooperation Process and Application Materials
A typical cooperation process is as follows:
- Initial contact: both parties communicate basic information via email, phone or online meetings.
- Document collection: potential distributors provide company qualifications, channel structure, key customers and team information.
- Market evaluation: ZODELO evaluates local market size and competitive landscape.
- Business negotiation: discussion of distribution level, authorized territory, sales targets and preliminary pricing policy.
- Contract signing: signing of distributor agreement and relevant compliance documents after terms are confirmed.
- Launch: completion of first order, training, and market plan, followed by official market launch.
We recommend preparing the following materials when applying (examples):
- Copy of business license and medical device distribution license (if applicable);
- Company profile and resumes of key management/sales team members;
- List of current agency brands and main product categories;
- List of major customers (sensitive information may be masked if needed, for reference only);
- Sales data of related products in the last 1–2 years (if available);
- Preliminary business plan for ZODELO in the local market.
Frequently Asked Questions
Q1: Does ZODELO always use exclusive distribution in every country?
A1: Usually we prefer to appoint one strong partner as the country/region master distributor in key markets. However, exclusivity is always tied to sales performance, compliance and other factors, and clearly defined in the contract.
Q2: Is the first order amount for a master distributor fixed?
Q3: Do you support OEM or private label cooperation?
A3: Yes. As long as local regulations are met and there is no conflict with ZODELO’s brand positioning, we can discuss OEM or private label projects. The specific terms will be laid out in a separate agreement.
Q4: Is there support for marketing and academic activities?
A4: Yes. ZODELO provides marketing support funds based on annual purchase volume, which can be used for academic conferences, patient education activities, online promotion and more. We can also offer materials and content suggestions.
Q5: What happens if a distributor fails to meet sales targets?
A5: We first work together with the distributor to analyze the reasons and adjust plans. If performance remains significantly below target across multiple assessment periods without reasonable improvement, ZODELO reserves the right to adjust the authorized territory or cancel exclusivity, as specified in the contract.
Partner with Zodelo — Your Reliable colostomy bag manufacturer
Zodelo understands the challenges distributors face. That’s why we’ve designed a full-service certified ostomy bag solution to simplify your procurement process. Elevate your project today!

